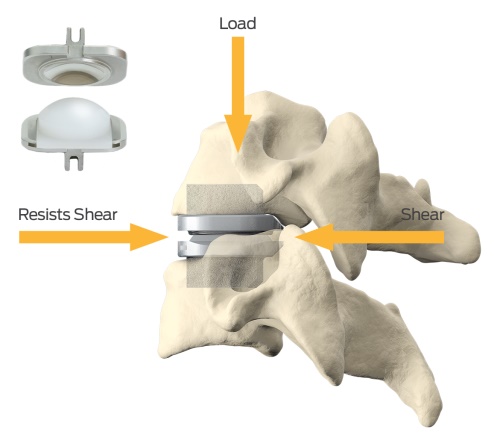
Artificial disc replacement surgery consists of removing a painful disc and replacing it with a artificial disc prosthesis that has similar biomechanical properties to a normal disc. Most artificial disc prostheses are made of metal and/or polymer. In general, the artificial disc replacement surgery is performed through a minimally invasive approach to the spine and patients recovery very quickly. However, it is important to note that artificial disc replacement surgery is still a major spinal reconstructive surgery and that results from FDA studies have been shown to be similar to fusion.